Pioneering world class science to treat ryanodine receptor (RyR)-related diseases
ARMGO Pharma is a privately owned, venture-backed, biotech company that is developing small molecule drugs that repair leaky Ryanodine Receptor (RyR) calcium channels associated with human diseases. We hold an exclusive, worldwide license from Columbia University for its RyR technology and know-how.
Our primary focus is on two orphan diseases: the life-threatening cardiac disease Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), and the severe muscle disease RYR1-Related Myopathy (RYR1-RM). The two conditions occur due to genetic mutations in the RYR genes. Mutations in RYR2 can lead to cardiac arrhythmias and sudden cardiac death, while mutations in RYR1 can cause muscle weakness.
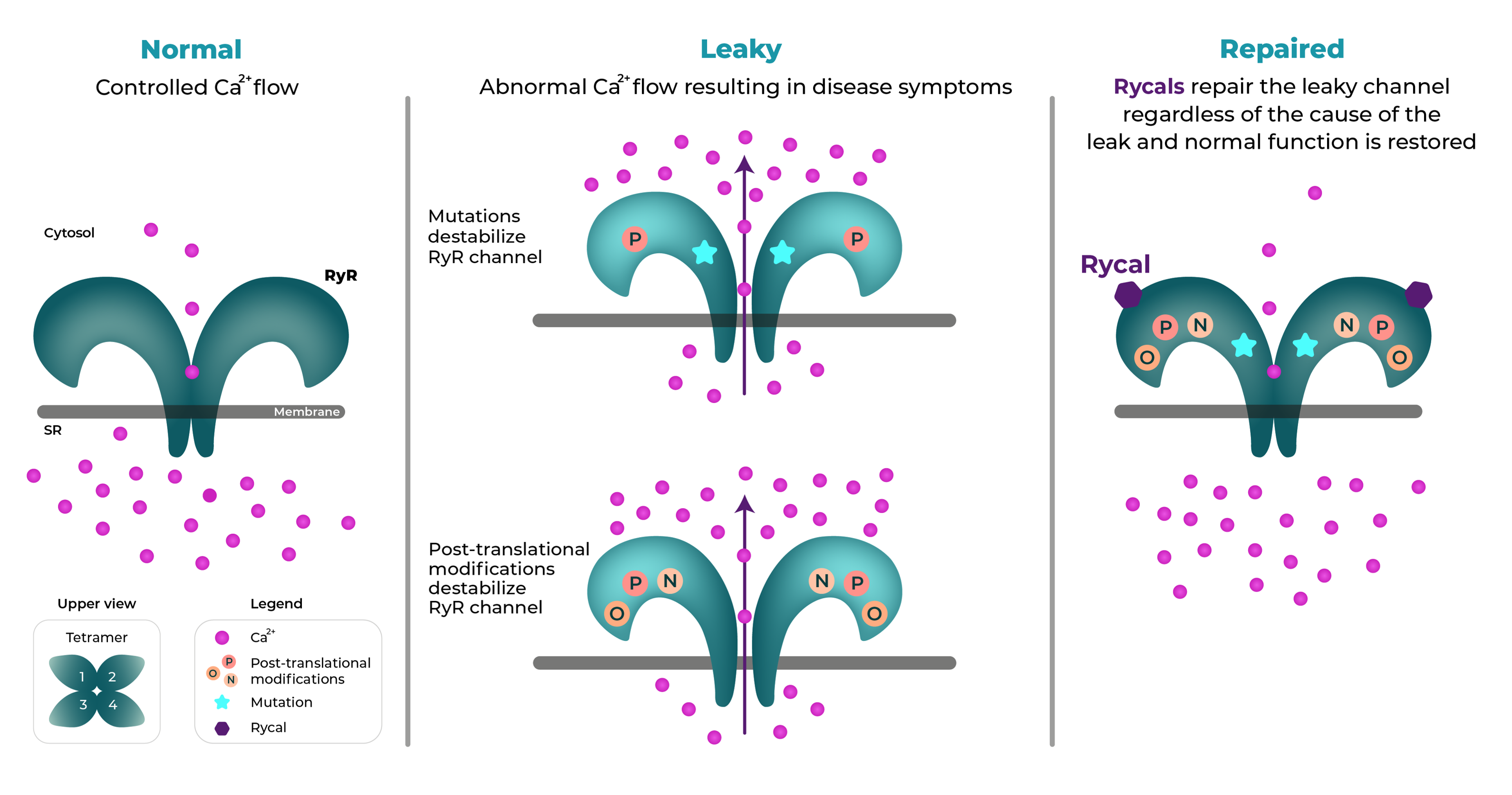
ARMGO’s molecules, termed Rycals® are designed to repair leaky RyR channels. Since the cause of the symptoms of CPVT and RYR1-RM is an abnormal calcium leak due to these mutations, Rycals® hold the promise of disease-modifying therapy for these two orphan genetic diseases. Beyond these genetic conditions Rycals® have therapeutic potential for other disorders associated with leaky RyRs.


The ARMGO Team


MD, PhD

PhD, JD

BBA

PhD


MD, PhD

PhD

PhD

MD

MD, PhD

MD

MD

JD

PhD

MD

PhD
Research
RyRs are homotetrameric intracellular calcium release channels responsible for Ca2+ flow from the sarcoplasmic/endoplasmic reticula (SR/ER) into the cytoplasm of most cell types. RyR1 is the predominant isoform in mammalian skeletal muscle, where SR Ca2+ release via RyR1 is required for excitation-contraction coupling and normal muscle function. RyR2 is the predominant isoform in cardiac muscle where ER Ca2+ release via RyR2 is required for normal cardiac muscle function. Human genetic mutations in RYR1 and RYR2 genes cause calcium to leak from RyR channels, leading to disease (Figure 1).
RyR channels normally alternate between a resting (closed) and excited (open) state. In certain diseases, RyR is modified and becomes leaky. Inhibition of the channel would stop the leak, but this intervention would not generally be beneficial since it would block the normal function of RyR. Rycals®, molecules that can restore normal channel function without blocking RyR, open the possibility of therapeutic interventions.
RyR1 and Skeletal Muscle
RyR1 plays a critical role in skeletal muscle, and RyR1 mutations in humans lead to a progressive myopathy, known as RYR1-RM. The causative mutations lead to a leaky channel, robbing the muscle of the ability to respond effectively to contraction signals, leading to muscle weakness.
RyR2 and Exercise-induced Sudden Cardiac Death
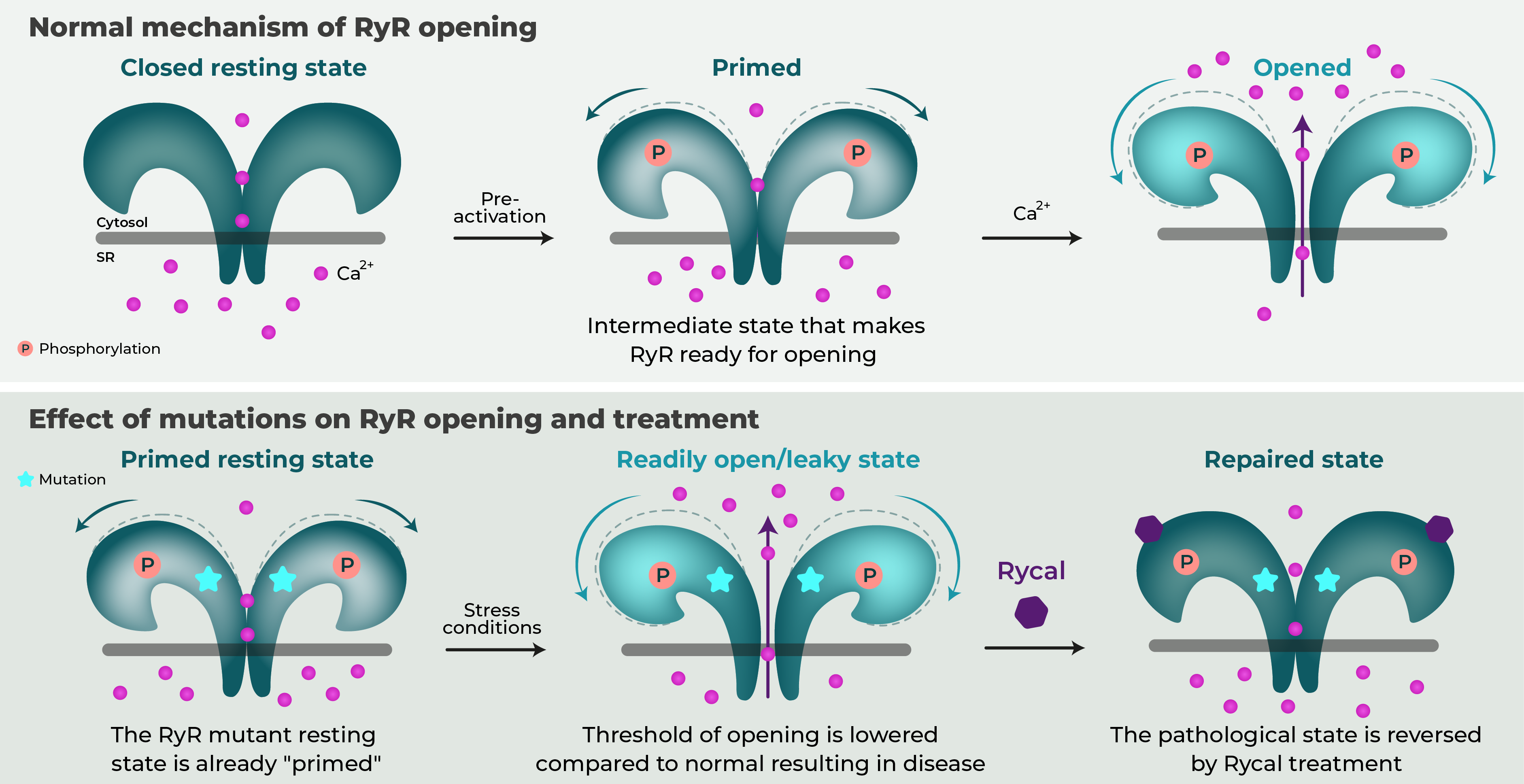
CPVT is characterized by exercise-induced fatal cardiac arrhythmias due to RyR2 mutations that render the channel leaky. Under normal states, the channel opening occurs during the contractile phase of the cardiac cycle, namely during systole. CPVT mutations render the channels leaky such that opening can occur inappropriately with exercise or stress leading to often fatal arrhythmias (Figure 2).
ARMGO has progressed Rycal® ARM210 into clinical development, with the goal of repairing leaky RyR channels in human diseases. Our focus is on the two human diseases with genetic mutations in RYRs, which validate RyR as a therapeutic target. The two diseases are the severe muscle disease RYR1-Related Myopathy (RYR1-RM) with mutations in RYR1, and the life-threatening cardiac disease Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), with mutations in RYR2 in most cases. In vitro, as well as in animal studies, ARM210 has been shown to be disease-modifying, by repairing the leaky channels and improving disease symptoms.
The mechanism by which ARM210 is believed to repair leaky channels has been elucidated by ARMGO’S founder, Dr. Andrew Marks (Columbia University). First, Melville et al., (2022) identified the binding site and mechanism of action of ARM210 on RyR1. A second study (Miotto et al., 2022) has recently delineated the structural basis for CPVT mutations in RyR2 and the mechanism of action of ARM210 on those mutant forms.
Using a series of cryo-electron microscopy structures, Miotto et al., showed that RyR2-R2474S, a mutant channel harboring a point mutation causing CPVT in humans, already adopts a primed state whereas wild-type RyR2 did not (Figure 2). The primed state has been shown to be a transition between the closed and open conformations of these channels. Once primed, the mutant channel can be more easily shifted to the open conformation. The normal channel will be open only during systole, which is the appropriate phase of the cardiac cycle for calcium release. The mutant channel however can be open in both systole and diastole with exercise and/or stress. This can lead to the ventricular arrhythmias characteristic of CPVT (Figure 2).
ARM210 was shown to bind and reverse the primed state of mutant RyR2. This binding stabilized the closed state and prevented pathological pore opening.
Clinical Studies
ARM210 is currently being investigated in both RYR1-RM and CPVT patients, to demonstrate proof-of-concept in both diseases.
Rycal S48168 (ARM210) for RYR1-related myopathies: a phase one, open-label, dose-escalation trial
Todd, Lawal, Chrismer, Kokkinis, Grunseich, Jain, Waite, Biancavilla, Pocock, Brooks, Mendoza, Norato, Cheung, Riekhof, Varma, Colina-Prisco, Emile-Backer, Meilleur, Marks, Webb, Marcantonio, Foley, Bönnemann, Mohassel. Eclinicalmedicine. 2024.
Targeting ryanodine receptors to treat human diseases
Andrew R Marks. J Clin Invest. 2023 PMID: 36647824
Miotto, Weninger, Dridi, Yuan, Liu, Wronska, Melville, Sittenfeld, Reiken, Marks. Sci Adv. 2022. PMID: 35857850
A drug and ATP binding site in type 1 ryanodine receptor
Melville, Dridi, Yuan, Reiken, Wronska, Liu, Clarke, Marks. Structure. 2022. PMID: 35580609
Intracellular calcium leak as a therapeutic target for RYR1-related myopathies
Kushnir, Todd, Witherspoon, Yuan, Reiken, Lin, Munce, Wajsberg, Melville, Clarke, Wedderburn-Pugh, Wronska, Razaqyar, Chrismer, Shelton, Mankodi, Grunseich, Tarnopolsky, Tanji, Hirano, Riazi, Kraeva, Voermans, Gruber, Allen, Meilleur, Marks. Acta Neuropathol. 2020. PMID: 32236737
Capogrosso, Mantuano, Uaesoontrachoon, Cozzoli, Guistino, Dow, Srinivassane, Filipovic, Bell, Vandermeulen, Massari, De Bellis, Conte, Pierno, Camerino, Liantonio, Nagaraju, De Luca. FASEB J. 2018. PMID: 29097503
Pipeline
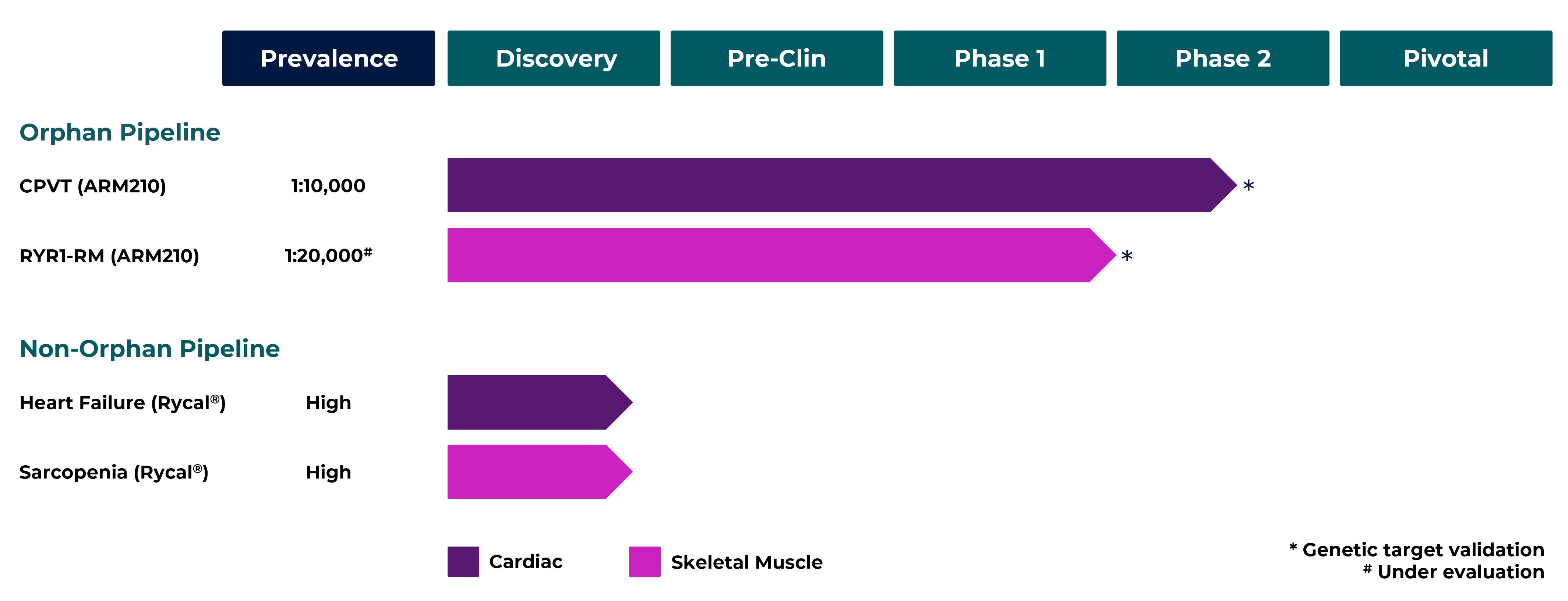
Clinical Trials
ARMGO is committed to bringing first-in-class and best-in-class therapeutics to patients with cardiac and skeletal muscle disorders. We are developing a novel class of compounds, Rycals®, which target and fix leaky Ryanodine Receptor (RyR) channels that cause human diseases.
Our clinical lead, ARM210 is currently being investigated in two RyR genetic disorders:
1. Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) – a rare condition causing life threatening changes in heart rhythm
ClinicalTrials.gov Identifier: NCT05122975
and
2. Ryanodine receptor 1-Related Myopathy (RYR1-RM) – a rare condition causing significant muscle weakness in adults and children.
ClinicalTrials.gov Identifier: NCT04141670
To follow our progress and news announcements please follow ARMGO Pharma Inc. on LinkedIn.


News & Events
ARMGO Pharma Discusses Results of RYR1-RM Phase 1b Trial of Rycal® ARM210 in Webinar by RYR1 Foundation

Contact
ARMGO Pharma, Inc.
923 Saw Mill River Road, PMB#260
Ardsley, NY 10502
USA
ARMGO Pharma, B.V.
Gooimeer 2-35
1411 DC Naarden
The Netherlands